DRUG ANALYSIS AND CONTROL
Section outline
-

This course for Drug Analysis and Control is designed for second-year Master's students in Pharmaceutical Chemistry, and is also suitable for those in Pharmaceutical Engineering and Pharmacy programs.
The content integrates fundamental chemical principles with their specialized applications to ensure the safety, efficacy, and quality of medicinal products. The knowledge presented in these chapters forms the essential foundation supporting the pharmaceutical industry's commitment to public health. Drawing on several years of teaching experience in this field, this COURSE aims to provide students with comprehensive support to master the essential concepts of pharmaceutical quality control.
This handout has been developed in accordance with the official program curriculum. It guides students from broad regulatory frameworks to the detailed application of analytical techniques for specific drug classes.
The essential context is first established in Chapters I and II, which examine the State System Structure in Quality Control and the rigorous preparation of technical documentation for standards. The core principles of pharmaceutical analysis are systematically presented in Chapter III, detailing specific characteristics, essential criteria, method validation parameters, and the general principles for establishing the authenticity of medicinal substances. Chapter IV discusses the types of technical analysis within a pharmaceutical complex. This theoretical foundation is complemented by the practical aspects covered in Chapter V: Sample Selection, emphasizing that the validity of any sophisticated analysis depends entirely on obtaining representative samples.
A comprehensive examination of modern pharmaceutical quality control methods is provided in Chapters VI through X. Chapter VI discusses sterilization methods for microbiological safety; Chapter VII presents ash analysis for purity assessment; Chapter VIII details physical characterization methods, including melting point and optical rotation determination; Chapter IX covers chemical analysis techniques through titrimetric methods and functional group identification; and Chapter X explores advanced physicochemical instrumentation, including spectral, chromatographic, and electrochemical techniques that form the core of modern quality control laboratories. Subsequent chapters (XI-XIV) apply these methods to specific drug classes, including aliphatic, aromatic, heterocyclic, and alkaloid compounds.
Upon completion of this course, students will be equipped not only to perform these analyses but also to critically evaluate analytical data, develop robust quality control protocols, and contribute to advancements in pharmaceutical sciences.
-

University: Djilali Bounaama Khemis Miliana
Faculty: Matter Sciences and Computer Science
Department: Chemistry
Specialization: Pharmaceutical Chemistry
Level: Master's Year 2
Module: DRUG ANALYSIS AND CONTROL
Semester: 2
Credits: 04
Unit Type: Fundamental
Coefficient: 02
Class Schedule: 1 hour 30 minutes, 3 sessions per week (2 lectures and 1 tutorial)Course Instructor: Dr. Meriem Fizir
Specialization: Pharmaceutical Analysis
Degree: Doctorate in Pharmaceutical Analysis
Academic Rank: MCA in Chemistry
Contact: You can contact me at meriem.fizir@univ-dbkm.dz from 6:00 PM onwards.Assessment Method:
Final assessment is conducted through:
-
Tutorials evaluation: Represents 33% of the final grade (12 points for presentations, 5 points for attendance, and 3 points for participation).
-
A final written exam: Counts for 67% of the final grade and covers all material taught in the course during the semester.
To pass the module, the overall average must be greater than or equal to 10 out of 20.
-
-
This first meeting with the instructor will be held online via Google Meet. The session will introduce the course, explain its objectives, and provide guidance on organization and evaluation. Attendance is strongly recommended to ensure a smooth start to the semester.”

-
-
PUT YOUR QUESTIONS HERE!
-
Table of Content
CHAPTER I: CONCEPT OF STATE SYSTEM STRUCTURE IN DRUG QUALITY CONTROL
CHAPTER II: QUALITY CONTROL OF DRUGS IN PHARMACIES
CHAPTER III : PARTICULARITIES OF PHARMACEUTICAL ANALYSIS AND THE ESSENTIAL CRITERIA
CHAPTER IV: TECHNICAL LABORATORY OPERATIONS IN A PHARMACEUTICAL COMPLEX
CHAPTER V : SAMPLING OPERATIONS
CHAPTER VIII: PHYSICAL ANALYSIS METHODS FOR CHEMICAL PREPARATIONS IN PHARMACY
CHAPTER IX: CHEMICAL ANALYSIS METHODS FOR CHEMICAL PREPARATIONS IN PHARMACY
CHAPTER X: PHYSICO-CHEMICAL ANALYSIS METHODS FOR DRUG QUALITY CONTROL
CHAPTER XI: ANALYSIS AND CONTROL OF ALIPHATIC PHARMACEUTICAL PREPARATIONS
CHAPTER XII: ANALYSIS AND CONTROL OF AROMATIC PHARMACEUTICAL PREPARATIONS
Chapter XIII: Analysis and Control of Heterocyclic Pharmaceutical Preparations
CHAPTER XIV: ANALYSIS OF ALKALOIDS
-

Upon successful completion of this course, the student will be able to:
-
Remember key regulatory frameworks and quality standards, including the roles of pharmacopoeias (Ph. Eur., USP, JP), ICH guidelines, and Good Manufacturing Practices (GMP).
-
Explain the fundamental principles and essential validation parameters (e.g., accuracy, precision, specificity) that ensure the reliability of pharmaceutical analytical methods.
-
Apply standard pharmacopoeial procedures to perform fundamental physical and chemical analyses.
-
Analyze complex pharmaceutical samples by selecting and utilizing appropriate chromatographic (e.g., HPLC, GC) and spectroscopic (e.g., UV-Vis, IR, AAS) techniques for separation and identification.
-
Differentiate between the roles and responsibilities of Quality Assurance (QA) and Quality Control (QC) and how they integrate within a pharmaceutical quality management system.
-
Evaluate the quality of raw materials, in-process samples, and finished products by interpreting analytical data against established specifications to make release or rejection decisions.
-
-


Based on the content and depth of the "Drug Analysis and Control" handout, a student would need a solid foundation in several core scientific disciplines to successfully understand the material. Here are the prerequisites for this course:
-
General and Organic Chemistry
-
A solid grasp of functional groups, acid-base chemistry, stoichiometry, and isomerism (especially stereochemistry) is fundamental. This is needed to understand drug structures, degradation pathways, and the chemical principles behind analytical reactions.
-
-
Analytical Chemistry Principles
-
Foundational knowledge of classical techniques like titrimetry and the basic concepts of instrumental analysis (e.g., calibration, separation science, spectroscopy) is crucial for understanding the modern methods (HPLC, GC, UV-Vis) that form the core of the course.
-
-
-
REGISTER YOUR ATTENDANCE HERE !
-
The concept of state system structure in drug quality control refers to the organization and management of quality control processes for drugs. This includes the establishment of protocols and procedures for testing, analysis, and evaluation of drug products to ensure that they meet the required standards for safety, efficacy, and quality. The state system structure may involve the use of various analytical techniques and technologies, such as chromatography, spectroscopy, and microbiological methods, as well as the implementation of quality management systems and regulatory frameworks to monitor and control drug quality throughout the entire supply chain. The ultimate goal of the state system structure in drug quality control is to safeguard public health by ensuring that drugs are safe, effective, and of high quality.
-


This chapter introduces the fundamental principles of quality control for drugs in pharmacies. It begins by defining key concepts like "quality" and "quality assurance" according to international standards. The chapter then explores the critical frameworks that ensure drug safety and efficacy, including Good Laboratory Practices (GLP) for research and the standards of the European Pharmacopoeia for quality control. Finally, it distinguishes between the roles of Quality Control (QC) and Quality Assurance (QA), highlighting their interconnected responsibilities in guaranteeing that every pharmaceutical product is effective, safe, and reproducible.
-
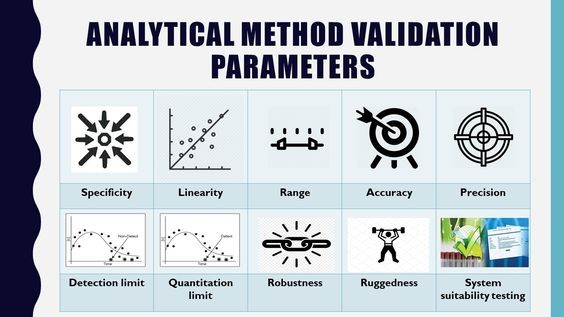
Pharmaceutical analysis involves the analytical signals treatment and transformation into data concerning the nature and amount of a substance, its chemical structure or spatial location of the sample - important components that require statistical treatment to have any real value. According to pharmacopoeial and GMP requirements, the analytical procedures used for drug quality control must be validated to confirm experimentally that they provide relevant and reliable information about the object of analysis and are suitable for their intended purpose. All quantitative tests, including impurities limit tests, should be validated, as should identification tests if it is necessary to confirm their specificity. The validation of analytical procedures is regulated by various national and international standards (ICH), which evaluate characteristics such as accuracy, precision, specificity, detection limit, quantitation limit, linearity, range, and robustness. Strict adherence to these validation parameters is essential to ensure the reliability and integrity of pharmaceutical analysis, which is critical for product quality, safety, and efficacy.
-
A Pharmaceutical Complex refers to an entire manufacturing plant or a network of facilities involved in producing drugs. "Technical Analysis" encompasses all the scientific testing and monitoring activities that verify every material and step in the process meets strict predefined standards. This is a legal and ethical requirement governed by Good Manufacturing Practices (GMP).
The core principle is: "Quality cannot be tested into a product; it must be built into it." Technical analysis provides the data to prove that quality has been built in at every stage.
-
Sampling comprises the operations designed to select a portion of a pharmaceutical product for a defined purpose. The sampling procedure should be appropriate to the purpose of sampling, to the type of controls intended to be applied to the samples and to the material to be sampled. All operations related to sampling should be performed with care, using proper equipment and tools. Any contamination of the sample by dust or other foreign material is liable to jeopardize the validity of the subsequent analyses.
-

Sterilization is a term referring to any process that eliminates (removes) or kills all forms of microbial life, including transmissible agents (such as fungi, bacteria, viruses, spore forms, etc.) present on a surface, contained in a fluid, in medication, or in a compound such as biological culture media. Sterilization can be achieved by applying heat, chemicals, irradiation, high pressure, or filtration.
-

Total ash and acid-insoluble ash are fundamental purity tests for pharmaceuticals and herbal medicines. While total ash measures all inorganic residues (both natural and contaminants), acid-insoluble ash specifically detects adulterants like silica or sand. This chapter covers the principles, standard methods, and step-by-step techniques to perform these analyses accurately.
-

Physical analysis methods form the first line of assessment in pharmaceutical quality control, providing fundamental, rapid, and often non-destructive means of evaluating chemical substances. These techniques focus on measuring the intrinsic physical properties of a material such as its color, density, melting point, and optical characteristics rather than its chemical reactions. This chapter details the standard procedures for these essential tests, which are critical for confirming identity, assessing purity, and ensuring batch-to-bustch consistency. From the visual assessment of a solution's colour to the precise determination of a compound's specific rotation, these methods are indispensable for verifying that raw materials and finished products comply with stringent pharmacopoeial standards before they are released for therapeutic use.
-
Physico-chemical analysis methods are essential tools in drug quality control, ensuring the safety, efficacy, and consistency of pharmaceutical products. These techniques evaluate critical attributes, including the identity, purity, strength, and stability of active ingredients and finished drugs. Ranging from classical titrimetry to advanced instrumental techniques, physico-chemical analysis forms the scientific foundation for guaranteeing that all medicines meet required quality standards. This chapter will focus on three pivotal instrumental categories: spectral, chromatographic, and electrochemical methods.
-

Pharmaceutical analysis relies heavily on chemical methods to ensure the identity, purity, quality, and stability of drugs. These methods, rooted in classical analytical chemistry, remain indispensable in modern laboratories due to their accuracy, cost-effectiveness, and alignment with global pharmacopeial standards like the USP (United States Pharmacopeia) and EP (European Pharmacopoeia). This chapter focuses on three foundational chemical techniques: titrimetric methods for quantification, the oxygen flask method for elemental analysis, and chemical identification tests for verifying substance identity.
-
Aliphatic compounds, characterized by carbon atoms arranged in straight or branched chains, or non-aromatic rings, form a foundational class of molecules in pharmaceutical science. Unlike their aromatic counterparts with rigid, delocalized electron systems, aliphatic molecules exhibit greater conformational flexibility, which directly influences their physicochemical properties, biological interactions, and analytical behavior. This category encompasses a vast range of substances, from simple hydrocarbon gases to complex macromolecules. The analysis of these preparations is critical, as their properties can dictate drug delivery, stability, and efficacy. This chapter is dedicated to the analytical strategies for four critically important sub-classes of aliphatic pharmaceuticals: alcohols, ethers, halogenated derivatives, and urea derivatives.
-
The analysis and control of aromatic pharmaceutical preparations is a critical discipline within pharmaceutical chemistry, dedicated to ensuring the safety, efficacy, and quality of these essential medicines. This field moves beyond simply confirming the identity of the active molecule to encompass a comprehensive assessment of its purity, stability, and final dosage form performance. This chapter is dedicated to the analytical strategies for three critically important sub-classes of aromatics pharmaceuticals: phenol, aniline, benzoic acid, and salicylic derivatives.
-

Typical alkaloids are derived from plant sources, they are basic, they contain one or more nitrogen atoms (usually in a heterocyclic ring) and they usually have a marked physiological action on man or animal.
-

The students are requested to prepare a PPT presentation on the following topics .
- Ash Analysis
- Analysis of Fats
- Analysis and control of antibiotics
- Analysis of salisilic acid and its derivatives
- Electrochemical methods
-
Opened: Monday, 15 December 2025, 12:00 AMDue: Monday, 22 December 2025, 12:00 AM
-
How clear were the course explanations?
-
References
1. Ahmed, R. (2024). High-Performance Liquid Chromatography (HPLC): Principles, Applications, Versatality, Efficiency, Innovation and Comparative Analysis in Modern Analytical Chemistry and In Pharmaceutical Sciences.
2. Arji, S. R., Vyshnavi, K., Sarella, P. N. K., & Mangam, V. T. (2024). Chromatographic techniques for pharmaceutical analysis. Futuristic Trends in Pharmacy & Nursing Volume 3 Book 19. Iterative International Publisher, Selfypage Developers Pvt Ltd, 163-172.
3. Cetina, M., Tranfić, M., Sviben, I., & Jukić, M. (2010). Synthesis, X-ray and spectroscopic analysis of some pyridine derivatives. Journal of molecular structure, 969(1-3), 25-32.
4. Colaço, R., & Serro, A. P. (2024). Sterilization methods. In Hydrogels for Tissue Engineering and Regenerative Medicine (pp. 139-159). Academic Press.
5. De, A., De, S., Saha, N., Das, B., Naskar, S., & Samanta, A. (2024). Pharmacopoeias, national formulary and extra pharmacopoeia. In Dosage Forms, Formulation Developments and Regulations (pp. 83-98). Academic Press.
6. Dey, P., Kundu, A., Kumar, A., Gupta, M., Lee, B. M., Bhakta, T., ... & Kim, H. S. (2020). Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). In Recent advances in natural products analysis (pp. 505-567). Elsevier. 7. Ghout, T. (2015). Maitrise de la libération pharmaceutique des lots de production industrielle (Doctoral dissertation, Université Toulouse lll-Paul Sabatier).
8. Goyal, K., Singh, N., Jindal, S., Kaur, R., Goyal, A., & Awasthi, R. (2022). Oxygen Flask Combustion Method. Advanced Techniques of Analytical Chemistry, 1, 113-119.
9. Hamad K. AbdulKadir .Volumetric Methods of Analysis Titrimetric Analysis.Course Handout.
10. Harvey, D. (2000). Modern analytical chemistry. McGraw Hill.
11. Islam, M. (2021). Study on the methods of the moisture content determination of active pharmaceutical ingredients recommended by the British pharmacopoeia (Doctoral dissertation, Brac University).
12. Kumar, R., Salwan, S., Kumar, P., Bansal, N., & Kumar, B. (2025). Electroanalysis Advances in Pharmaceutical Sciences: Applications and Challenges Ahead. Analytica, 6(2), 12.
13. Lyle, R. E., & Lyle, G. G. (1964). A brief history of polarimetry.
REFERENCES
14. Mahendra, D. R., Al Khadir, M. K., & Muzzamil, A. R. (2025). Quality Control and Quality Assurance in Pharmaceutical Industry. International Journal of Food Safety and Public Health, 12(1), 1-12. 15. Marjanović-Balaban, Ž., Jelić, D., Antunović, V., & Gojković, V. (2013). Determination of water content in pharmaceutical substances. Journal of Hygienic Engineering and Design, 137-141. 16. MIRI Faïza. ENREGISTREMENT D’UN MÉDICAMENT GÉNÉRIQUE FABRIQUÉ EN ALGÉRIE. ASPECTS TECHNICO-RÉGLEMENTAIRES DU CONTRÔLE DE QUALITÉ. Mémoire Master. 2014.
17. Nally, J. D. (2016). Good manufacturing practices for pharmaceuticals. CRC Press. 18. Organisation mondiale de la Santé (2010). Règles OMS de bonnes pratiques applicables par les laboratoires de contrôle qualité pharmaceutique.
19. Pedersen, O. (2006). Pharmaceutical chemical analysis: methods for identification and limit tests. CRC Press.
20. Pleteneva, T. V., Morozova, M. A., Uspenskaya, E. V., & Khatchaturyan, M. A. (2019). Drugs quality control (Theoretical foundation and practical application): The Coursebook.
21. Rao, B. S. (2017). A study on Ash values and pharmacopoeial assay methods in herbal pharmaceuticals. Pharma View, 93-7. 22. Ritgen, U. (2023). Atomic absorption spectroscopy (AAS). In Analytical Chemistry I (pp. 247-253). Berlin, Heidelberg: Springer Berlin Heidelberg.
23. Sarr, D. M. Mise en place des bonnes pratiques de laboratoire dans une structure de recherche.
24. Sharma, A. Fundamentals of Mass Spectrometry in Chemical Analysis. FUNDAMENTAL CONCEPTS OF SPECTROSCOPY AND SPECTROMETRY FOR CHEMICAL ANALYSIS, 49. 25. Sheinin, E. B. (2025). Pharmacopeial methods and tests. Specification of drug substances and products, 161-184.
26. Shepherd, A. J. (1999). Good laboratory practice in the research and development laboratory. Gene Therapy Technologies, Applications and Regulations: From Laboratory to Clinic, 375-381. 27. Siddiqui, M. R., AlOthman, Z. A., & Rahman, N. (2017). Analytical techniques in pharmaceutical analysis: A review. Arabian Journal of chemistry, 10, S1409-S1421.
28. Siddiqui, M. R., AlOthman, Z. A., & Rahman, N. (2017). Analytical techniques in pharmaceutical analysis: A review. Arabian Journal of chemistry, 10, S1409-S1421. 29. Synthesis Of Heterocyclic Compounds In Pharmaceutical Industry. (2025). African Journal of Biomedical Research, 28(2S), 1597-1604. 30. Ward, M., & O'Boyle, N. M. (2025). Analysis of the structural diversity of heterocycles amongst European medicines agency approved pharmaceuticals (2014–2023). RSC Medicinal Chemistry, 16(10), 4540-4570.
31. World Health Organization. (2006). The international pharmacopoeia. World Health Organization.
32. World Health Organization. (2007). Quality assurance of pharmaceuticals: a compendium of guidelines and related materials. Good manufacturing practices and inspection (Vol. 2). World Health Organization. 33. Xu, Z. (2022, March). Considerations on Regulatory Quality Control in Pharmaceutical Industry. In 7th International Conference on Economy, Management, Law and Education (EMLE 2021) (pp. 324-328). Atlantis Press.


 EVALUATE YOUR KNOWLEDGE BY PASSING THIS TEST.
EVALUATE YOUR KNOWLEDGE BY PASSING THIS TEST.




 Upon the completion of this chapter, the student will be able to
Upon the completion of this chapter, the student will be able to
 Upon completing this chapter, the student will be able to:
Upon completing this chapter, the student will be able to: 

 Upon the completion of this course, the student will be able to
Upon the completion of this course, the student will be able to Upon completing this chapter, th
Upon completing this chapter, th
