T. Analyse 1
Section outline
-

-
Preface
-
-
Main Objectives of the Course
- Introduce students to the fundamental principles of physico-chemical analysis techniques used to separate, identify, and quantify chemical species.
- Develop the ability to apply different separation methods (filtration, extraction, distillation, chromatography, etc.) to practical laboratory problems.
- Strengthen theoretical understanding and analytical skills by connecting experimental methods with their underlying physical and chemical principles.
- Prepare students for advanced studies and laboratory practice by fostering autonomy, accuracy, and critical thinking in chemical analysis
-
-
§ Faculty: Matter and Computer Sciences Faculty
§ Target Audience: L2 Chemistry
§ Course Title: Physico-Chemical Analysis Techniques I
§ Semester: 03
§ Teaching Unit: Discovery Unit – UD3
§ Credits: 2
§ Coefficient: 02
§ Duration: 15 weeks
§ Classroom: S9
§ Instructor: KHERROUBI SEDDIK
§ Evaluation: Continuous assessment: 33% – Final exam: 67%
§ Contact: k.seddik@univ-dbkm.dz
-
YOU MUST GET MORE THAN 50% OF THE POINTS TO ACCEPT THIS COURSE
-
-

-
This first meeting with the instructor will be held online via Google Meet. The session will introduce the course, explain its objectives, and provide guidance on organization and evaluation. Attendance is strongly recommended to ensure a smooth start to the semester.”
-
-

-
Course Content
CHAPTER I: General Overview of Separation Methods
· Definition and importance of separation in chemistry.
· Separation of components from heterogeneous mixtures.
o Solid–liquid mixtures: filtration, decantation, centrifugation.
o Liquid–liquid systems: separation of immiscible liquids (separating funnel, decantation).
· Treatment of homogeneous phases (physical and chemical techniques).
CHAPTER II: Separation by Phase Manipulation
· Separation from liquid solutions.
· Precipitation and elimination techniques.
· Salting-out method: principle, mechanism, and applications.
CHAPTER III: Extraction by Chemical Reactions
· Principles of separation based on chemical transformations.
· Examples: selective precipitation, ion exchange, complexation.
· Advantages and limitations.
CHAPTER IV: Extraction with an Immiscible Solvent
· General principles of liquid–liquid extraction.
· Partition law and Nernst distribution law.
· Partition coefficient and distribution ratio.
· Expression of extraction yield.
· Simple extraction: definition, quantitative study, and practical implementation.
· Multiple extractions and optimization strategies.
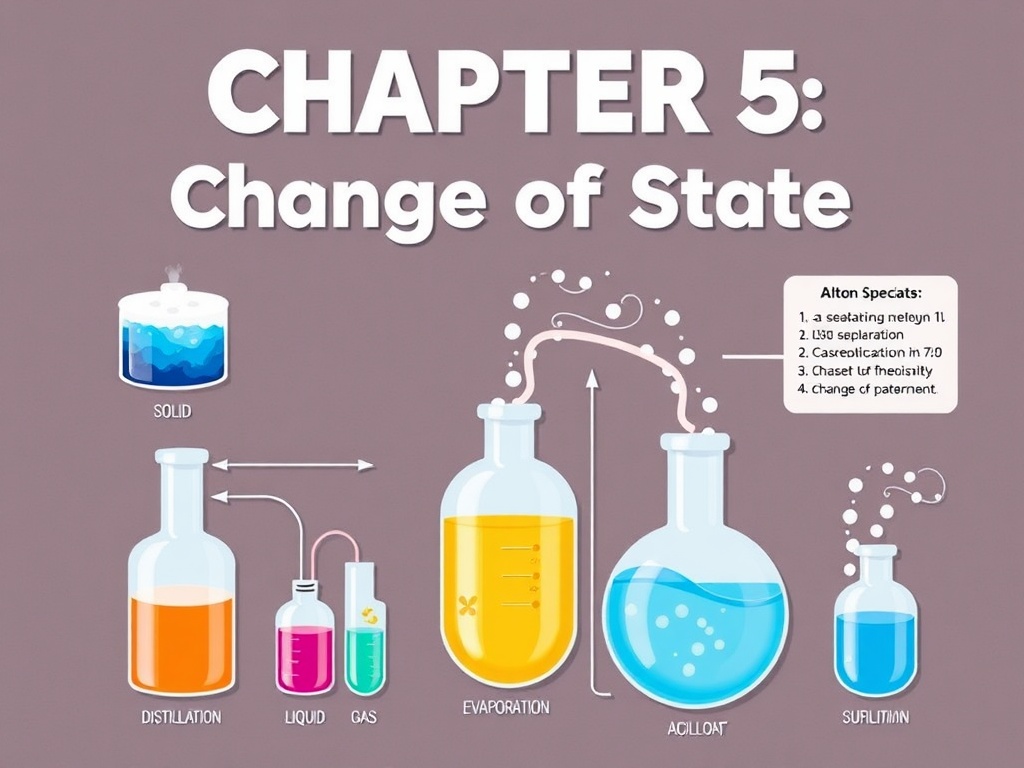
CHAPTER V: Separation by Change of State
· General reminders on phase changes.
· Sublimation: principle and applications.
· Distillation techniques:
o Simple distillation.
o Fractional distillation (rectification).
o Distillation of immiscible liquids (azeotropic and steam distillation).
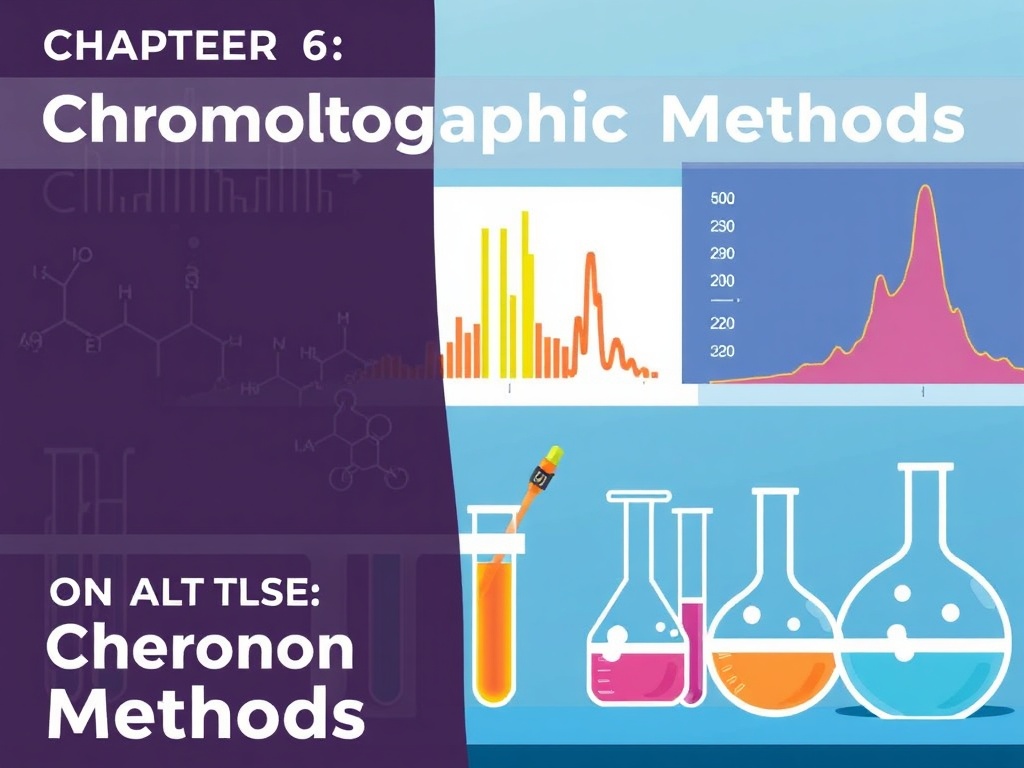
CHAPTER VI: Chromatographic Methods
· General principles and classification of chromatographic techniques.
· Schematic representation and interpretation of a chromatogram.
· Theoretical approaches:
o Plate theory.
o Peak shape and symmetry.
o Adsorption and partition phenomena.
· Kinetic theory of chromatography: H.E.T.P and Van Deemter equation.
· Practical applications:
o Thin Layer Chromatography (TLC).
o High Performance Liquid Chromatography (HPLC).
o Gas–Liquid Chromatography (GLC).
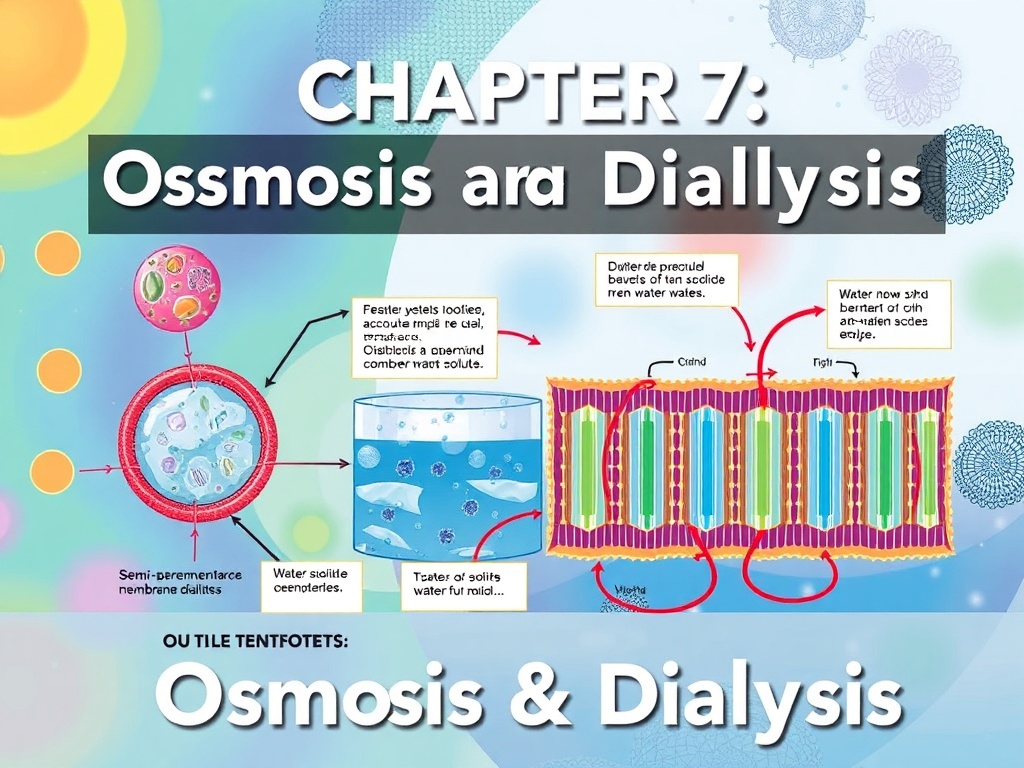
CHAPTER VII: Osmosis & Dialysis
· Osmosis: definition, principles, and applications in separation.
· Dialysis: principle, mechanisms, and uses in analytical chemistry and biochemistry.
-
-
This chapter introduces the basic principles of separating mixtures, focusing on mechanical and physical methods such as filtration, centrifugation, and liquid-liquid separation.

-
-
Poser une question sur une partie que vous n’avez pas bien comprise.
-
-
Continuous assessment: 50% of the final grade
-
Students learn how to induce phase separation in solutions using techniques like elimination and salting-out to isolate desired compounds.

-
download
-
Répondre à la question d’un autre étudiant si vous en êtes capable
-
The test score represents 50% of the final exam
-
-
This section covers chemical reactions used to selectively extract certain components from mixtures, based on reactivity

-
download
-
Poser une question sur une partie que vous n’avez pas bien comprise
-
The test score represents 50% of the final exam
-
-
Focuses on solvent extraction principles, explaining partitioning, distribution coefficients, and practical applications in quantitative extraction processes

-
download
-
Poser une question sur une partie que vous n’avez pas bien comprise
-
The test score represents 50% of the final exam
-
-
This chapter explores methods that rely on changes of physical state, such as sublimation and distillation, to separate mixtures with different volatilities
-
download
-
Poser une question sur une partie que vous n’avez pas bien comprise.
-
The test score represents 50% of the final exam
-
-
Students study chromatographic techniques, including their theoretical foundations and practical implementations, with emphasis on separation efficiency and applications in analytical chemistry
-
download
-
download
-
download
-
Give one practical example of chromatography application (in the laboratory, pharmaceutical industry, quality control, etc.)
-
The test score represents 50% of the final exam
-
-
This final chapter introduces membrane-based separation techniques, highlighting the principles of osmosis and dialysis for purifying or concentrating solutions
-
download
-
Ask one question about a concept that you find unclear or interesting to explore further.
-
Opened: Wednesday, 17 September 2025, 12:00 AMDue: Wednesday, 24 September 2025, 12:00 AM
Complete these exercises and send them in PDF forma
-
How clear were the course explanations?

-
-

-
References:
-