DRUG ANALYSIS AND CONTROL
Section outline
-
-
University: Djilali Bounaama Khemis Miliana
Faculty: Science and Technology
Department: Material Sciences
Specialty: PHARMACEUTICAL CHEMISTRY
Level: Master 2 Pharmaceutical Chemistry
Module: Drug Analysis and Control
Semester: 03
Coefficient: 03
Lecturer: Dr. Fizir Meriem.
specialty: Pharmaceutical Analysis
Diploma: Doctor in pharmaceutical analysis
Grade: MCA
Contact: You can contact me on meriem.fizir@univ-dbkm.dz from 6 a.m.
Assessment method: The final evaluation is carried out through a final exam.
To pass the module, the general average must be greater than or equal to 10 out of 20
-
-

- Physicochemical analysis methods
-
-
-

Objectives
· Exploring the state system structure in drug quality control
· Examining quality management systems in the pharmaceutical industry
· Understanding the role of pharmacopoeias
· Developing knowledge of quality documentation and standards
-
-
-
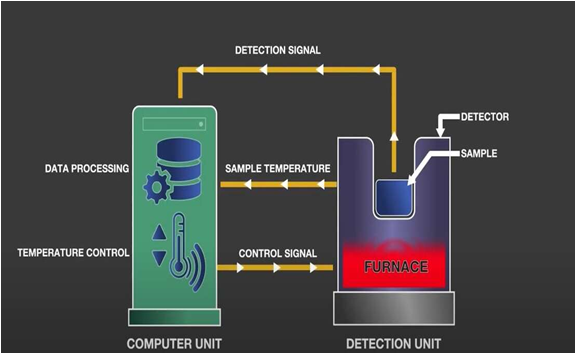
Objective
To study pharmacopoeial methods of thermal analysis used for the quality control of API and drugs.
-
-
-

OBJECTIVE
To create a system of knowledge about the optical pharmacopoeial methods of medicines quality control on the example of polarimetry and Refractometry.
-
-
-
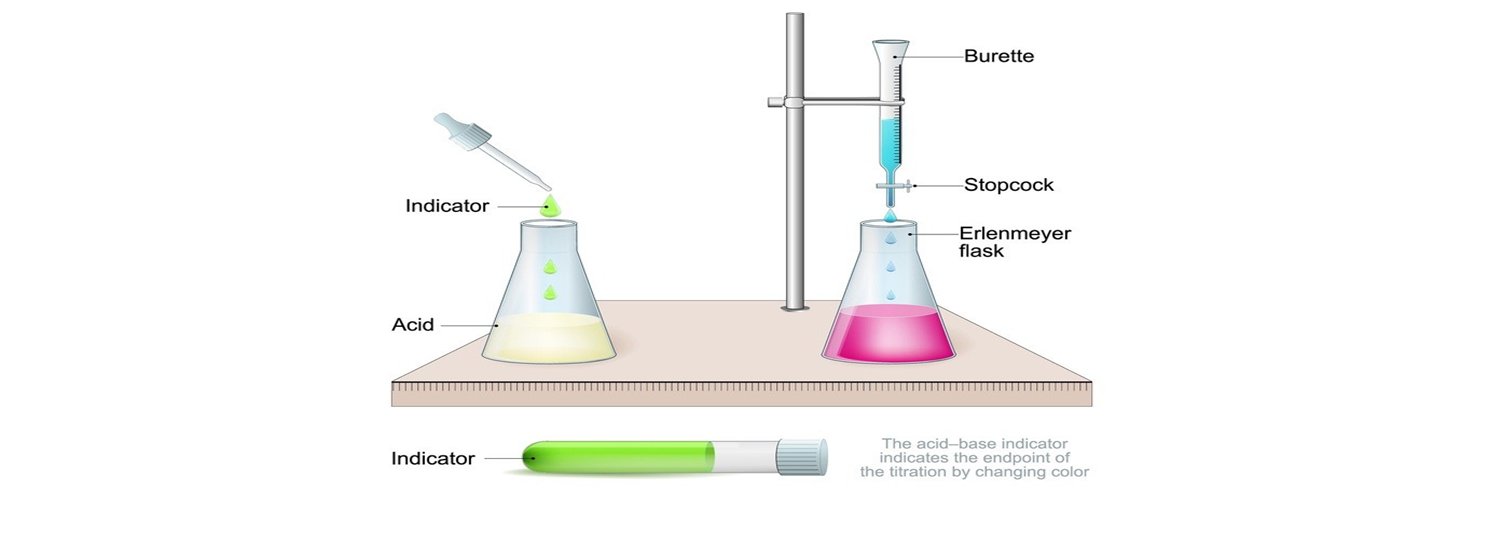
Objective
Form a system of knowledge about the titrimetric pharmacopoeial methods for drugs quality control.
-
-
-
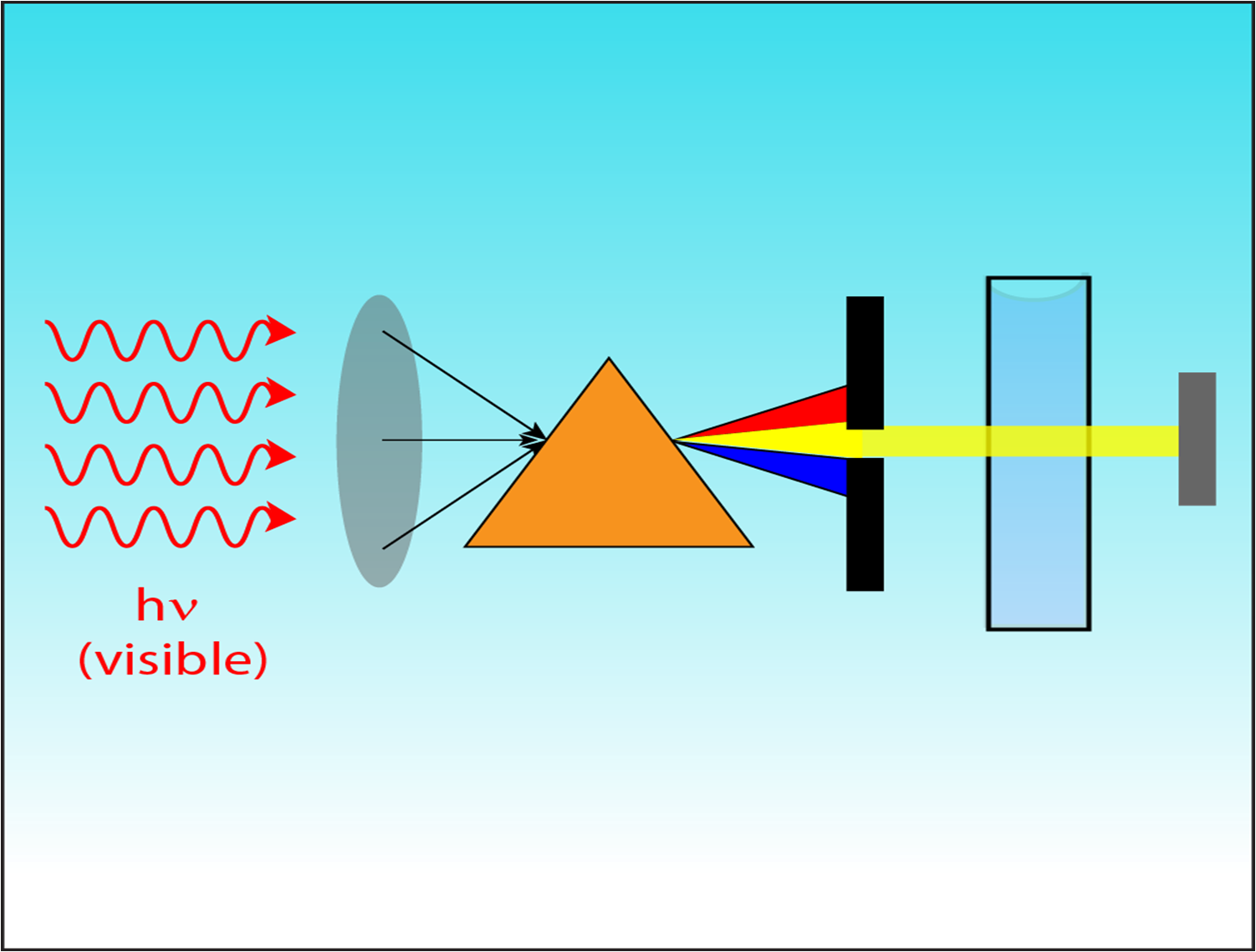
Objective:
to learn the theoretical and practical foundations of ultraviolet and visible spectroscopy; assess the quality of medicine using UV/visible spectroscopy.
-
-
-
References
1. Pleteneva, T. V., Morozova, M. A., Uspenskaya, E. V., & Khatchaturyan, M. A. (2019). Drugs quality control (Theoretical foundation and practical application): The Coursebook. Open Textbook Library.
2.Good Manufacturing Practice (GMP):
US Food and Drug Administration (FDA). Current good manufacturing practice (CGMP) regulations. Accessed August 2, 2023. https://www.fda.gov/drugs/pharmaceutical-quality-resources/current-good-manufacturing-practice-cgmp-regulations
3.World Health Organization (WHO). Good manufacturing practices for pharmaceutical products: main principles. WHO Technical Report Series, No. 908, Annex 2. Accessed August 2, 2023. https://www.who.int/medicines/areas/quality_safety/quality_assurance/control_laboratories/WHOTRS908Annex2.pdf
4. ISO 9001:
International Organization for Standardization (ISO). ISO 9001:2015 Quality management systems -- Requirements. Accessed August 2, 2023. https://www.iso.org/standard/62085.html
5. Pharmacopeial standards:
United States Pharmacopeia (USP). General chapter <41> Balances. Accessed August 2, 2023. https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/generalChapter41.pdf
European Pharmacopoeia (Ph. Eur.). Quality of pharmaceuticals. Accessed August 2, 2023. https://www.edqm.eu/en/european-pharmacopoeia-ph-eur-10th-edition-0
6.Siddiqui, M. R., AlOthman, Z. A., & Rahman, N. (2017). Analytical techniques in pharmaceutical analysis: A review. Arabian Journal of chemistry, 10, S1409-S1421.
7.Xu, Z. (2022, March). Considerations on Regulatory Quality Control in Pharmaceutical Industry. In 7th International Conference on Economy, Management, Law and Education (EMLE 2021) (pp. 324-328). Atlantis Press.
8.Force, A. T. G. T. (2016). QUALITY CONTROL.
9. FARHAT, Kulsoom. Pharmaceutical Quality Assurance.
10.LYLE, Robert E. et LYLE, Gloria G. A brief history of polarimetry. 1964.
-